How Many Nonpolar Bonds Does Ch3oh Contain
CH3 OH and H2O 2. If you want to calculate the mass of Methanol then it is here.

Ch3oh Lewis Structure Geometry Hybridization And Polarity Techiescientist
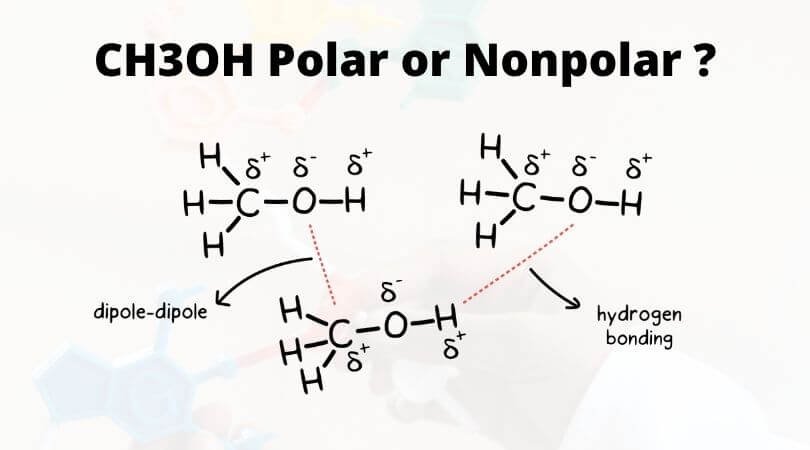
To summarise CH3OH is a polar and a neutral compound that has a tetrahedral geometry and bent and tetrahedral shapes with respect to the oxygen and carbon atoms respectively.
. A sample of C3H8O that contains 200. Breaking to the American market. Ch3oh polar or nonpolar.
The CF bond is slightly more polar than the CH bond. O2 LiCl Br2 CH3OH Like dissolves like. Learn to determine if CH3OH Methanol is polar or non-polar based on the Lewis Structure and the molecular geometry shapeWe start with the Lewis Structur.
How many non-polar bonds does CH3OH contain. Its structure can be thought of as very similar if not exactly to the water molecule and the CH3- group decreases the. The chemical formula of Methanol is CH3OH.
CH 3 CH 3. A 770 kJ b 856 kJ c 518 kJ d 326 kJ e 428 kJ e None of the above. CH4O or CH3OH is methanol and is polar covalent compound.
CH3OH H and OH I know i have to get an equation having. How many non-polar bonds does CH3OH contain. CH3OH and H2O 4.
How many polar bonds does CH3F have. CH3OH Bond angles The central Carbon atom forms four bonds in the compound three with the Hydrogen atom and one with the hydroxyl OH group. It has three C-H sigma nonpolar bonds one C-O sigma polar bond and one O-H sigma polar bond along with the hybridization of sp3.
Octane CH3CH2CH2CH2CH2CH2CH2CH3 non polar C. It works well to remove oil by. Is CH3OH polar or non polar.
Generally substances with a dielectric constant of less than 15 are considered non-polar. In this case hydrogen bonding does NOT occur since the F atom is bonded to the central C atom F must be bonded to H in order for hydrogen bonding to occur. Is CH3OH Polar or Nonpolar.
Due to this NN N N is a non-polar bond. That is polar compounds usually are soluble in water and non-polar compounds are not soluble in water. CHCl3 CH4 CH3OH CH3F HINT.
CH3CH2CH2OH CH3CH2OH CH3OH CH3CH2CH2CH2OH. Its chemical formula is CH3OH containing one carbon atom one oxygen and four hydrogen atoms. Which one of the following is most soluble in hexane C6H14.
How Many Non Polar Bonds Does Ch3oh Contain How Many Non Polar Bonds Does Ch3oh Contain - All through the eighties Hyundai saw rapid advancement making sizeable inroads into global markets. The molecular mass of CH3OH is 3204 g per mol. Chemistry questions and answers.
Assuming that there is an adequate supply of phosphorus atoms how many P2O5 molecules can be made from 20 molecules of O2. Which molecule contains polar covalent bonds and non-polar covalent bonds. Arrange the following compounds in order of increasing solubility in water.
In non-polar covalent bonds the electrons are shared equally. And as this carbon atom has an sp3 hybridization and forms a tetrahedron shape it has the bond angles of 1095 degrees with its bonding atoms. Both the atoms are the same in the molecule with no electronegativity difference so electrons are shared equally by the nitrogen atom.
6 sigma and 2 pi. Note that hexane is non polar. Methanol is the simplest alcohol that contains only 1 carbon atom connected with three hydrogen bonds and one hydroxyl group.
According to the lewis structure of methanol it has one O-H bond three C-H bonds and one C-O bond. Answer 1 of 6. Draw the lewis structure of the molecule C3H4 How many sigma and pi bonds does it contain.
Molecules contains _____ carbon atoms. Hence the molecule dinitrogen does not contain polar bonds. Florine lithium What is the Lewis diagram for carbonic acid H2CO.
CH3F Contains four polar bonds but their polarities are of different magnitude. A H-0-0-0-H 0 b H-0-0-0. The molecular mass of methanol is 3204 g per mol.
Calculate the molarity of a solution that contains 0300 moles of CrBr in 0600 L of water. How many polar bonds does CH4 contain. Anything with 2N2 H2 O2 CO2 Cl2.
This is also known as methyl alcohol. How many non-polar bonds does CH3OH contain. 4 How many non-polar bonds does CH3OH contain.
What is the name of this compound. Methanol is its IUPAC name. Methanol with a dielectric constant of about 33 would therefore be considered polar water with a dielectric constant of about 88 is even more polar.
3 Fluorine F forms a binary ionic compound with lithium Li. 1 mol mass of C 4 mol mass of H 1 mol mass of O 12 1 4 16 32grams. Answer to Is CCl2H2 polar or non polar.
There are 2 lone pairs on oxygen atom. Based off molecular polarity determined by VSEPR theory in which solvent would glucose be the least soluble. Is methanol and ionic compound.
CH3F H is not bonded to F so not hydrogen. Which of the following can hydrogen bond. CH3O H and H2O 3.
To begin with methanol H_3C-OH is asymmetrical so it could not be nonpolar being 100 symmetrical in all directions means all dipole moments would cancel out completely. In a solution prepared by mixing CH3OH with H2O the major species pesent are 1. CH3OH contains one carbon atom four hydrogen atoms and one hydroxyl group in its molecule.
If so what is your answer. There are total of 14 electrons in valence shells in the overall molecule as lone pairs and bonds. Methanol is comprised of hydrgen.
On the other hand until 1986 the company realized considered one of its key goals. Contains 4 bonds but no net polarity. CH3OH is a polar molecule owing to the polarity rendered by the -OH group.
Methanol CH 3 OH is the simplest alcohol which has only one carbon atom. Soap had an ionic and nonpolar end.

Ch3oh Lewis Structure Geometry Hybridization And Polarity Techiescientist

Ch3oh Polar Or Nonpolar Methanol Polarity Geometry Of Molecules
Comments
Post a Comment